#OTD A Year Ago, Moderna’s RNA Vaccine Became The First #COVID19 Vaccine To Enter Phase 1 Trials. The

#OTD a year ago, Moderna’s RNA vaccine became the first #COVID19 vaccine to enter phase 1 trials. The latest #ChemVsCOVID graphic with the Royal Society of Chemistry takes a brief look at how prior research helped COVID vaccines reach this point quickly: https://ift.tt/3cE5xHR https://ift.tt/3rV4v0F
More Posts from Amateurchemstudent and Others
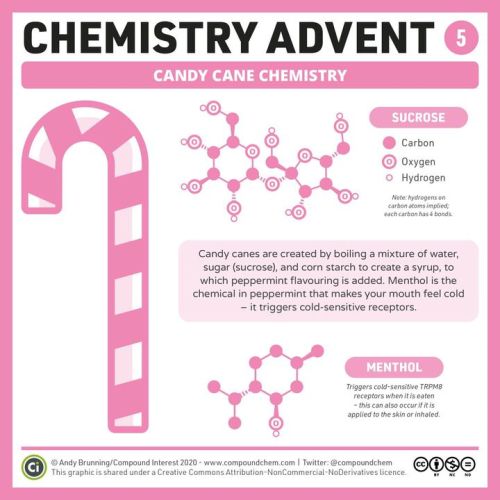
It’s day 5 of #ChemAdvent – here’s why peppermint candy canes make your mouth feel cold! bit.ly/chemadvent2020 https://ift.tt/2JM6bZ7
Shapes of Molecules
his post is more information than trying to explain something - the truth is, you just need to learn shapes of molecules like you do with anything. I’ve got a physical chemistry mock tomorrow that I’m dreading since I’ve done zero revision. The fact that I run a study blog yet don’t revise myself is odd, but what else can I do? Oh, wait … revise. So here it is, my last minute revision for myself and you too - I present, shapes of molecules!
VSEPR stands for valence shell electron pair repulsion theory. If you’ve ever seen a moly-mod or a diagram of a molecule in 3D space, you may wonder how they decided it was that shape. Well, VSEPR answers all.
The theory essentially states that electron pairs are arranged to minimise repulsions between themselves - which makes sense, since electrons carry the same charge and therefore try to repel each other. Of course, there are different types of electron pairs, lone and bonding. The strongest repulsions happen between lone pair - lone pair followed by lone pair - bonding pair and finally, bonding pair - bonding pair have the least repulsion.
Since the repulsion governs the shape of the molecule, to work out a molecule’s shape you must look at dot and cross diagrams or electron configurations to see how a molecule is bonded. There are many methods to do this, but the bottom line is that you must work out how many bonding pairs of electrons and how many lone pairs are involved.
The easiest shape to learn is linear. This has two bonding pairs and no lone pairs at an angle of 180 degrees, since that is the furthest the two can get away from each other. Examples of linear molecules include carbon dioxide and beryllium chloride.


Next up is trigonal planar. This has three bonding pairs and no lone pairs, each at the angle of 120 degrees. Trigonal means three and planar means on one plane, this should help you in identifying the molecules since after a fourth pair of electrons, the shape becomes 3D. Examples of trigonal planar molecules include boron trifluoride and sulfur trioxide.


What if you were to have two bonding pairs and two lone pairs? Well, then you’d have a bent molecule. Water is a good example of a bent molecule. Since it has two lone pairs that repel the other two bonding pairs more than they repel each other, the bond angle is 104.5. I’d be careful though, as in many textbooks it shows a bent molecule to have one lone pair and a different bond angle.

Another variation of the bent molecule I’ve seen is the one with two bonding pairs and one lone pair. It is deemed as bent with a bond angle of 109 or sometimes less than 120 degrees.

Tetrahedral molecules have four bonding pairs and no lone pairs. The bond angle is 109.5 degrees. Examples of this include the ammonium ion, methane and the phosphate ion. A good thing to note here is how these molecules are drawn. To demonstrate the 3D shape, where the molecule moves onto a plane, it is represented with a dashed line and triangular line along with a regular straight line.


Trigonal pyramidal, sometimes just called pyramidal, is where there are three bonding pairs and a lone pair. Bond angles are roughly 107 degrees due to the repulsion from the lone pairs. An example of a trigonal pyramidal molecule is ammonia, which has a lone pair on the nitrogen.

Having five bonding pairs gives a trigonal bipyramidal structure. I guess the three bonding pairs on the trigonal plane accounts for that part of the name, where the rest comes from the position of the remaining two. These molecules have no lone pairs and have a bond angle of 90 degrees between the vertical elements and 120 degrees around the plane. Diagrams below are much clearer than my description! Examples of this include phosphorus pentachloride.


Six bonding pairs is an octahedral structure. I know this is confusing because octahedral should mean 8 but it’s one of those things we get over, like the fact sulfur isn’t spelt with a ph anymore. It’s actually to do with connecting the planes to form an octahedral shape.There are no lone pairs and each bond angle is a nice 90 degrees. Common examples include sulfur hexafluoride.


Square planar shapes occur when there are six bonding pairs and two lone pairs. All bond angles are 90 degrees! They take up this shape to minimise repulsions between electrons - examples include xenon tetrafluoride.

The final one to know is T-shape. This has three bonding pairs and two lone pairs. These molecules have bond angles of (less than) 90 degrees, usually a halogen trifluoride like chlorine trifluoride.

There are plenty more variations and things you could know about molecular geometry, but the truth is, there won’t be an extensive section on it. It’s a small part of a big topic!
I’m not going to do a summary today since I’d just be repeating the same information (I tried to keep it concise for you guys) so instead I’ll just leave you with,
Happy studying!

The UK’s academic pipeline is failing to retain Black, Asian and other ethnic minority chemists, an analysis by the Royal Society of Chemistry’s Inclusion and Diversity team has shown. The figures are particularly stark for Black students, who are far less likely than white students to be pursuing a PhD and higher academic positions.
While the numbers of UK domiciled ethnic minority students entering chemistry degrees mirror the general population, the figures change dramatically as these students advance through academia’s career stages. At undergraduate, Asian students are around 14% of the population, dropping to 7% at postgraduate. For Black students, the drop is even more severe, from about 5% at undergraduate to just over 1% at postgraduate.
‘Beyond the PhD, the numbers absolutely diminish to the very senior levels of academia, where it is essentially barren ground for Black chemists,’ comments Robert Mokaya, who works on sustainable energy materials at the University of Nottingham. ‘When I was promoted in 2008, I was very aware that there was a lack of others like me but was unaware that I was possibly the first Black chemistry professor in the UK,’ says Mokaya. ‘My hope then was that there would be others. But I don’t know of any other appointment since then. And of course that is really very disappointing.’
Continue Reading.


Enthalpy - a thermodynamic property
When I first learned about enthalpy, I was shocked - it felt more like a physics lesson than a chemistry lesson. The thought of learning more about thermodynamics than my basic understanding from my many science lessons in lower school made me bored out of my mind. But enthalpy is actually pretty interesting, once you get your head around it…
Reactions which release heat to their surroundings are described to be exothermic. These are reactions like combustion reactions, oxidation reactions and neutralisation reactions. Endothermic reactions take in heat from their surroundings, such as in thermal decomposition. Reversible reactions are endothermic in one direction and exothermic in the other.
These facts are important when you start to look at enthalpy. Enthalpy is basically a thermodynamic property linked to internal energy, represented by a capital H. This is pretty much the energy released in bond breaking and made in bond making. We usually measure a change in enthalpy, represented by ∆H. ∆H = enthalpy of the products (H1) - enthalpy of the reactants (H2). This is because we cannot measure enthalpy directly.
In exothermic reactions, ∆H is negative whereas in endothermic reactions, ∆H is positive.
∆H is always measured under standard conditions of 298K and 100kPa.
In reversible reactions, the ∆H value is the same numerical value forwards and backwards but the sign is reversed. For example, in a forward exothermic reaction, the ∆H value would be -ve but in the backwards reaction (endothermic) the ∆H would be +ve.
Reaction profiles are diagrams of enthalpy levels of reactants and products in a chemical reaction. X axis is enthalpy rather than ∆H and the Y axis is the progress of reaction, reaction coordinate or extent of reaction. Two horizontal lines show the enthalpy of reactants and products with the reactants on the left and the products on the right. These should be labelled with their names or formulae.
In an endothermic reaction, product lines are higher enthalpy values than reactants. In an exothermic reaction, product lines are lower enthalpy values than reactants. The difference between product and reactant lines is labelled as ∆H. Values are measured in kJ mol-1.
Reaction pathways are shown with lines from the reactants to the products on enthalpy level diagrams. This shows the “journey” that the enthalpy takes during a reaction. They require an input of energy to break bonds before new bonds can form the products. The activation energy is the peak of the pathway above the enthalpy of reactants. It is the minimum amount of energy that reactants must have to react.

Standard enthalpy values are the ∆H values for enthalpy changes of specific reactions measured under standard conditions, represented by ⊖. There are three of these:
1. Standard enthalpy of reaction ( ΔHr⊖ )
The enthalpy change when substances react under standard conditions in quantities given by the equation for the reaction.
2. Standard enthalpy of formation ( ΔfH⊖ )
The enthalpy change when 1 mole of a compound is formed from its constitutent elements with all reactants and products in standard states under standard conditions.
The enthalpy of formation for an element is zero is it is in it’s standard state for example, O2 enthalpy is zero.
3. Standard enthalpy of combustion ( ΔcH⊖ )
The enthalpy change when 1 mole of a substance is burned completely in excess oxygen with all reactants and products in their standard states under standard conditions.
Values for standard enthalpy of formation and combustion must be kept to per mole of what they refer.
Summary
Reactions which release heat to their surroundings are described to be exothermic. Endothermic reactions take in heat from their surroundings, such as in thermal decomposition.
Reversible reactions are endothermic in one direction and exothermic in the other.
Enthalpy is a thermodynamic property linked to internal energy, represented by a capital H. We usually measure a change in enthalpy, represented by ∆H.
∆H = enthalpy of the products (H1) - enthalpy of the reactants (H2). We cannot measure enthalpy directly.
In exothermic reactions, ∆H is negative whereas in endothermic reactions, ∆H is positive.
∆H is always measured under standard conditions of 298K and 100kPa.
In reversible reactions, the ∆H value is the same numerical value forwards and backwards but the sign is reversed.
Reaction profiles are diagrams of enthalpy levels of reactants and products in a chemical reaction. They
In an endothermic reaction, product lines are higher enthalpy values than reactants. In an exothermic reaction, product lines are lower enthalpy values than reactants.
The difference between product and reactant lines is labelled as ∆H.
Values are measured in kJ mol-1.
Reaction pathways are shown with lines from the reactants to the products on enthalpy level diagrams. They plot enthalpy against reaction progress.
Reactions require an input of energy to break bonds before new bonds can form the products. The activation energy is the peak of the pathway above the enthalpy of reactants. It is the minimum amount of energy that reactants must have to react.
Standard enthalpy values are the ∆H values for enthalpy changes of specific reactions measured under standard conditions, represented by ⊖.
Standard enthalpy of reaction ( ΔHr⊖ ) is the enthalpy change when substances react under standard conditions in quantities given by the equation for the reaction.
Standard enthalpy of formation ( ΔfH⊖ ) is the enthalpy change when 1 mole of a compound is formed from its constitutent elements with all reactants and products in standard states under standard conditions.
The enthalpy of formation for an element is zero is it is in it’s standard state.
Standard enthalpy of combustion ( ΔcH⊖ ) is the enthalpy change when 1 mole of a substance is burned completely in excess oxygen with all reactants and products in their standard states under standard conditions.
Values for standard enthalpy of formation and combustion must be kept to per mole of what they refer.
Happy studying!
🌻 little habits/things to do more of 🌻
dailies
make your bed. (no, really.)
set your top 3 to-dos for the day.
do your top 3 to-dos for the day. (heh)
stretch.
unpack your bag when you get home.
prepare your things for the next day before sleeping.
skincare. (your basic cleanse and moisturize)
sweep the floor of your bedroom.
talk to your plants. (if you have plants)
update your financial report/expense tracker.
take a good photo.
meditate.
hug at least three people. (seriously.)
weeklies
polish your school shoes.
mop your bedroom floor.
dare i say, laundry. (don’t put it off!)
exfoliate.
take a leisure walk.
review your past week and plan your next week accordingly. (a part of your routine may not be working–try something new)
make a piece of art. (a sketch, a collage, a quote in pretty lettering, a god-awful poem..)
sanitize your gadgets. (whip out the wet tissue and wipe away at your phone, your laptop, your mouse, your earphones–just don’t forget to IMMEDIATELY follow that up with a dry cloth to prevent fogging and short circuits)
watch a TED Talk.
make a new playlist.
monthlies
wash your bag.
wash your shoes.
change the sheets of your bed and your pillows.
clip your nails. (honestly)
wax/shave. (if you want. i just really like how fresh my skin feels after i torture it with razors and wax strips)
wipe your shelves/the tops of your furniture clean. (try to avoid dusting. it just scatters the dirt everywhere. use a damp cloth instead)
see if there’s anything in your storage that you don’t need/want anymore and give stuff away or sell them.
review your month and plan the next one accordingly. (just like your weeks. remember to refer to your Life Goal/Year’s Goals page)
finish reading at least one book. (and review it!)
discover new songs.
- 🍂
Biochemistry
Update: Pictures are working!
Atoms
There are a few basic chemistry concepts that are essential to understand. For starters, understanding what an atom is and its basic properties.
Atoms are the building block of all matter. They have a positive nucleus, with positive protons, and neutral neutrons. In a large area surrounding the nucleus, is the electron cloud, made of negatively charged electrons.
An atom in its elemental state is always neutral.
When an element has a charge, it is because it has an unequal number of protons an electrons, making it an ion. Sometimes an element’s nucleus has an unequal number of neutrons and protons, making it an isotope. Carbon-14, for example, has 8 neutrons, instead of the 6 that Carbon-12 has. Carbon-14 is also a radioisotope, meaning it emits particles and decays at a rate called a half-life, making it useful for fossil dating. Along with that, radioactive carbon can be used as a tracer. This means it is incorporated in CO2 molecules and used to track metabolic pathways.
The location of the electron affects how the atom will react with other elements. When electrons are in the lowest available energy level, they are in the ground state. When they absorb energy, they move to a higher energy level, entering the excited state. For instance, when chlorophyll absorbs light energy, electrons within it are boosted to higher energy levels. This provides the energy necessary to produce sugar when they return to their ground state level as they release the energy they absorbed.
Bonding
Elements bond when two nuclei are attracted to each other. Energy is released when a bond is formed. All atoms want to either get rid of all their electrons on their outer shell or fill their outer shell with 8 (or in hydrogen’s case, 2) electrons, which makes them stable. There are 3 kinds of bonds, but for biochemistry, Ionic and covalent bonds are what is relevant.
Ionic bonds form ions (hence the name.) They occur when electrons are transferred. The atom that gains electrons becomes a negatively charged anion. The atom that loses electrons becomes a positively charged cation.
Covalent bonds are made when electrons are shared. This occurs when the two atoms have electronegativities that are closer together than in an ionic bond. Electronegativity is the tendency of an atom to pull electrons towards it. These bonds can be polar if the electronegativity is high enough. A polar molecule is a molecule with a partial charge. For example, water is a polar molecule, as oxygen is extremely electronegative, and water is partially electronegative.

Hydrogen Bonding
Hydrogen bonding is a specific kind of intermolecular force that is essential to life. It is what keeps the 2 strands of DNA bonded together, and gives water its unique characteristics. Since oxygen has a partial negative charge, and hydrogen has a partial positive charge, they are naturally drawn to each other.

Hydrophobic vs Hydrophilic
Polar molecules are hydrophilic. This is because they are attracted to the partially charged ends of water. Hydrophilic means they are attracted to water. (Not in that way… sick) NaCl or table salt is hydrophilic. This is why salt dissolves in water.
Non-polar molecules are hydrophobic. This means they are repelled by water. (They’re filthy water haters.) Lipids are hydrophobic, which is why fats and oils do not dissolve in water.
The cell membrane is a phospholipid bilayer, only allowing nonpolar substances to dissolve through it. Large polar molecules have to use specific hydrophilic channels.
Characteristics of Water
Water is a unique molecule, and without its unique properties, life on earth would not exist as it does, or even at all.
Water has a high specific heat: Because hydrogen bonds are so strong, it requires a lot of heat energy to break them. This is why large bodies of water remain the same temperature, and why coastal cities have a consistent temperature because the water absorbs all the heat energy before it can warm up.
Water has a high heat of vaporisation: A large amount of energy is needed for water to vaporise, which is why sweating is such an effective cooling method.
Water has high adhesion properties: Adhesion is when one substance clings to another. Adhesion causes capillary action, which occurs in the xylem of plants, and is used to bring water up from the roots without expending energy.
Water is a universal solvent: Due to its high polarity, water makes an excellent solvent.
Water is extremely cohesive: Molecules of water tend to stick to each other. This is observed in surface tension and allows for small insects to run across the surface of the water. Cohesion is also necessary to bring water up from the roots, by transpirational-pull cohesion tension.
Ice is less dense than water: Instead of freezing all the way through, ice crystallises, leaving large amounts of space, causing ice to float. This is essential for the survival of marine life during the winter, as they can live beneath the ice.
pH
pH is calculated by taking the -log of the chance of finding hydronium (H30+) ions within a certain amount of water. Hydronium is made in rare circumstances, where a hydrogen ion breaks off from a water molecule. Normally, there is a 1 in 10 million chance of there being a hydronium ion. This is the equivalent of 1x10^-7. The -log of this number is 7, the neutral pH.
Any pH below 7 is acidic. Any pH above 7 is basic. Stomach acid has a pH of 2, while bleach has a pH of 11. Human blood has a pH of around 7.4
Most living cells need to have an internal environment with a pH of around 7. Buffers exist to regulate pH by either absorbing excess hydrogen ions or donating missing hydrogen ions. In human blood, the bicarbonate ion (HCO3) is essential.
Macromolecules
There are 4 types of macromolecules: carbohydrates, lipids, proteins, and nucleic acids.
Carbohydrates
Carbohydrates are made of carbon, hydrogen, and oxygen. They supply quick and easy energy. 1 gram of all carbohydrates will release 4 calories of energy. In our diet, they can be found almost everywhere in foods such as rice, pasta, bread, cookies, etc.
There are 3 kinds of carbohydrates: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides
All monosaccharides have a chemical formula of C6H12O6. It is the placement of the carbon, oxygen, and hydrogen that determines its properties. Glucose, fructose, and galactose are all examples. They are isomers, meaning they have the same chemical formula, but a different structure.

Disaccharides
When 2 monosaccharides join together, they create disaccharides. They all have the chemical formula C12H22O11. Dehydration synthesis is the process that creates them. This process releases 1 molecule of water, hence the name. Lactose, maltose, and sucrose are all examples.
Hydrolysis is the exact opposite of dehydration synthesis. It is used during digestion. One molecule of water is used to breakdown polymers into monomers.
Polysaccharides Polysaccharides are long polymers of carbohydrates. Cellulose (plant cell wall), chitin (exoskeleton, fungi cell wall), glycogen (how animals store carbohydrates) and starch (how plants store carbohydrates) are all examples.
Lipids
Lipids include fats, oils, and waxes. Most contain 1 glycerol and 3 fatty acids. Glycerol is alcohol.

Fatty acids are the building blocks of lipids and are hydrocarbon chains with carboxyl groups at the end. There are 2 varieties; saturated and unsaturated. (3 if you count trans-fats when extra hydrogen is added to the fat to make the lipid solid)
Saturated fats are solid at room temperature, and are famously unhealthy as they are linked to heart disease.
Unsaturated fats are liquid at room temperature and are good dietary fats.

Lipids store much more energy than carbohydrates. 1 gram of any lipid will release 9 calories of heat per gram. They can be structural, as in the phospholipids of the cell membrane, or they can be hormones.
Proteins
Proteins are polymers of amino acids linked together by peptide bonds.
Amino acids are identifiable by their carboxyl group, amine group, and variable R, attached to a central carbon atom.
Proteins are complex and perform a vast array of duties, such as growth and repair, being enzymes, membrane channels, and hormones.
1 gram of protein releases 4 calories of heat.
Proteins contain the elements C H O N P S
There are only 20 amino acids coding for the thousands of proteins in the human body.
Protein Structure
There are 4 levels to the structure of a protein.
The primary structure results from the sequence of amino acids making up the polypeptide
The secondary structure results from hydrogen bonding within the molecule. This causes a helical structure
The tertiary structure is an intricate 3-dimensional shape or conformation of a protein and most directly decides the function of the protein. Enzymes denature in high temperatures or in the wrong pH because the tertiary structure is compromised.
The quaternary structure is only found in proteins that have more than 1 polypeptide chain, such as in haemoglobin.

Enzymes
Enzymes are large proteins
Enzymes lower the energy of activation, speeding up the reaction, as it lowers the amount of energy needed to start the reaction.
The chemical an enzyme works on is known as a substrate
Enzymes are specifically designed for specific substrates. For example, lactase only works on lactose. Notice the naming pattern for enzymes and their substrates.
The induced fit model is an explanation for how they work. When the substrate enters the active site, it induces the enzyme to change its shape to fit the substrate.
Enzymes can be reused as they do not degrade during a reaction
Enzymes are assisted by cofactors (minerals) or coenzymes (vitamins)



Prions
Prions are proteins that cause diseases. Mad cow disease is an example. It is a misformed protein able to influence other proteins to fold in the same way.
Nucleic Acids
There are 2 kinds of nucleic acids: RNA and DNA. They are necessary for carrying genetic information.
Nucleic acids are polymers of nucleotides
The nucleotides are the two purines: Adenine and Guanine, and the 3 pyrimidines, Thymine, Uracil, and Cytosine. Uracil is only found in RNA, and thymine is only found in DNA. Adenine connects with thymine/uracil, and guanine connects with cytosine.

The two kinds of water

Credit: University of Basel
Pre-sorted ortho-water and para-water molecules with differently oriented nuclear spins (blue or red arrows) react with diazenylium ions (centre left) at different speeds.
–
Researchers from the University of Basel’s Department of Chemistry, Switzerland, has investigated how the two forms of water differ in terms of their chemical reactivity – the ability to undergo a chemical reaction. Both forms have almost identical physical properties, which makes their separation particularly challenging.
It is less well-known that water exists in two different forms (isomers) at the molecular level. The difference is in the relative orientation of the nuclear spins of the two hydrogen atoms. Depending on whether the spins are aligned in the same or opposite direction, one refers to ortho- or para-water.
The was made possible by a method based on electric fields. Using this, researchers were able to initiate controlled reactions between the pre-sorted water isomers and ultracold diazenylium ions (protonated nitrogen) held in a trap. During this process, a diazenylium ion transfers its proton to a water molecule. This reaction is also observed in the chemistry of interstellar space.
It was discovered that para-water reacts about 25% faster than ortho-water. This can be explained in terms of the nuclear spin also influencing the rotation of the water molecules. As a result, different attractive forces act between the reaction partners. Para-water is able to attract its reaction partner more strongly than the ortho-form, which leads to an increased chemical reactivity.
-
 carljparker liked this · 3 years ago
carljparker liked this · 3 years ago -
 1biomed reblogged this · 3 years ago
1biomed reblogged this · 3 years ago -
 alexmck liked this · 4 years ago
alexmck liked this · 4 years ago -
 chicannaxo liked this · 4 years ago
chicannaxo liked this · 4 years ago -
 waterstudios liked this · 4 years ago
waterstudios liked this · 4 years ago -
 chatelaine-panda liked this · 4 years ago
chatelaine-panda liked this · 4 years ago -
 lycragym85 liked this · 4 years ago
lycragym85 liked this · 4 years ago -
 jamie-rubbish-gi liked this · 4 years ago
jamie-rubbish-gi liked this · 4 years ago -
 pleasurehunter2000 liked this · 4 years ago
pleasurehunter2000 liked this · 4 years ago -
 eternallyyours666 liked this · 4 years ago
eternallyyours666 liked this · 4 years ago -
 jaemhan liked this · 4 years ago
jaemhan liked this · 4 years ago -
 assiduousaditi liked this · 4 years ago
assiduousaditi liked this · 4 years ago -
 not-the-chosen1 reblogged this · 4 years ago
not-the-chosen1 reblogged this · 4 years ago -
 iammte reblogged this · 4 years ago
iammte reblogged this · 4 years ago -
 kristinalakana liked this · 4 years ago
kristinalakana liked this · 4 years ago -
 ericalagu liked this · 4 years ago
ericalagu liked this · 4 years ago -
 themoonsacademia liked this · 4 years ago
themoonsacademia liked this · 4 years ago -
 amateurchemstudent reblogged this · 4 years ago
amateurchemstudent reblogged this · 4 years ago -
 amateurchemstudent liked this · 4 years ago
amateurchemstudent liked this · 4 years ago -
 engineeringworldhealth reblogged this · 4 years ago
engineeringworldhealth reblogged this · 4 years ago -
 engineeringworldhealth liked this · 4 years ago
engineeringworldhealth liked this · 4 years ago -
 pixaldiary liked this · 4 years ago
pixaldiary liked this · 4 years ago -
 striker0111 liked this · 4 years ago
striker0111 liked this · 4 years ago -
 ffbuddy68 liked this · 4 years ago
ffbuddy68 liked this · 4 years ago -
 barbedwireandfences liked this · 4 years ago
barbedwireandfences liked this · 4 years ago -
 sparklemiranda liked this · 4 years ago
sparklemiranda liked this · 4 years ago -
 turalati04 liked this · 4 years ago
turalati04 liked this · 4 years ago -
 jwacademy reblogged this · 4 years ago
jwacademy reblogged this · 4 years ago -
 quornonthequob liked this · 4 years ago
quornonthequob liked this · 4 years ago -
 crossedwithblue liked this · 4 years ago
crossedwithblue liked this · 4 years ago -
 triffebayarea liked this · 4 years ago
triffebayarea liked this · 4 years ago -
 fremen liked this · 4 years ago
fremen liked this · 4 years ago -
 dogadaev liked this · 4 years ago
dogadaev liked this · 4 years ago -
 istabee liked this · 4 years ago
istabee liked this · 4 years ago -
 noumaios34 liked this · 4 years ago
noumaios34 liked this · 4 years ago -
 wolfofromania liked this · 4 years ago
wolfofromania liked this · 4 years ago -
 evakkorotta reblogged this · 4 years ago
evakkorotta reblogged this · 4 years ago
